Case Presentation:
You are working in the CCU overnight when you get a call about a 70-year-old man with unknown past medical history presenting with a progressive history of intermittent lightheadedness, fatigue, and occasional near-syncope specifically during exertion. He came in tonight after passing out while he was driving. He does not smoke, drink, or do any drugs.
Vitals: BP 90/50 mmHg, HR 40 bpm, RR 14, Temp 36.8°C, SpO2 98% on room air
Ask Yourself:
How do you differentiate between the types of bradycardia and how do you work it up?
When do we get concerned about bradycardia
What do we do in the acute setting (pharm vs transvenous / transcutaneous pacing) vs long term?
When do people get devices? What type of devices do they usually get?
Normal sinus rhythm with rates in the 40s. Normal axis. PR interval notably prolonged. No ST elevations /depressions or T wave inversions.
Differentiating Types of Bradycardia
When patients have bradycardia (HR <60 bpm), it is usually due to:
Sinoatrial (SA) node dysfunction (aka sick sinus syndrome)
Atrioventricular (AV) block
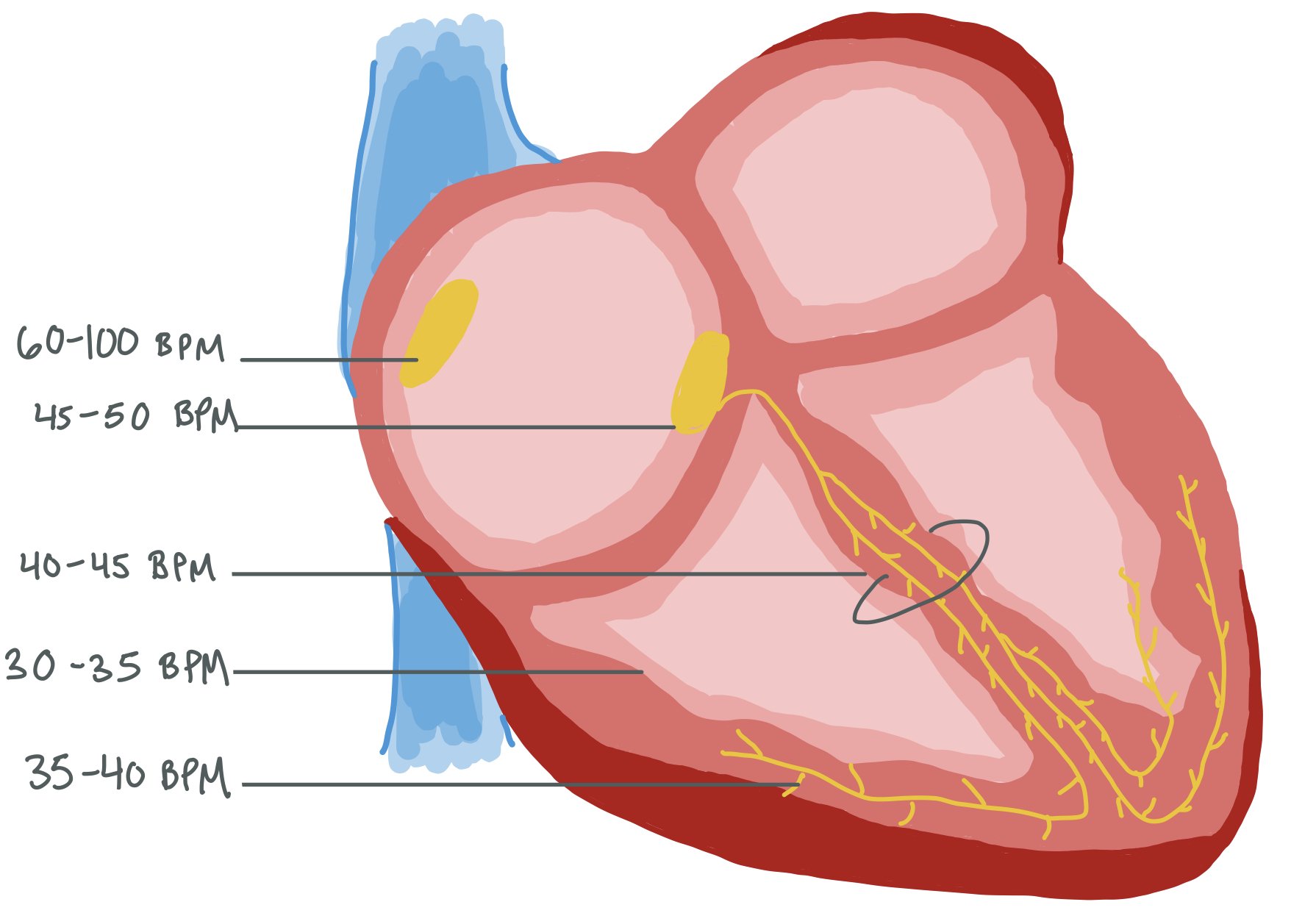
The picture above shows different parts of the conduction system, and issues can occur throughout. Specifically, these abnormalities can occur in the sinus node, atrial tissue, AV node, and the Bundle of His to cause many types of arrhythmias, especially bradycardia.
Sinoatrial Node Dysfunction (SND)
Sinus Bradycardia
This is a regular (albeit SLOW) rhythm originating from the sinoatrial (SA) node.
The key mechanism involves an increased parasympathetic (vagal) tone or intrinsic dysfunction of the SA node, leading to delayed depolarization or suppressed automaticity of pacemaker cells. In many cases, this is due to age dependent progressive fibrosis of the sinus node. Other causes can include ischemia or ion channel abnormalities within the SA node itself.
Often seen in athletes or during sleep.
May result from medications (e.g., beta-blockers, calcium channel blockers), hypothyroidism.
Sick Sinus Syndrome (SSS)
Dysfunction of the SA node causing inappropriate bradycardia, sinus pauses, and tachycardia. When the bradycardia and tachycardia occur close together, this is called “tachy-brady syndrome.”
Often results from progressive fibrosis and degeneration of the SA node and surrounding atrial tissue, which impairs impulse generation and propagation.
Common in elderly patients.
Atrioventricular (AV) Block
First-Degree AV Block
Pathophysiology: The impulse from the SA node is delayed at the AV node but eventually reaches the ventricles.
Mechanism: Often due to increased vagal tone, beta-blockers, calcium channel blockers, or fibrosis of the AV node.
Electrocardiogram (ECG): Prolonged PR interval (>200 ms) without dropped beats.
Second-Degree AV Block
Mobitz Type I (Wenckebach)
Mechanism: Progressive fatigue of AV nodal conduction, leading to increasing PR intervals until one impulse fails to conduct.
Causes: Increased vagal tone, inferior myocardial infarction, or drugs that affect the AV node.
Location: Usually within the AV node (benign prognosis).
Mobitz Type II
Mechanism: Intermittent failure of conduction without PR interval prolongation.
Location: The pathology is usually in the His-Purkinje system (distal to AV node).
Clinical Significance: More serious and may progress to complete heart block. Mobitz Type II is commonly seen in structural heart disease, fibrosis, or infarction of conduction tissue.
Third-Degree AV Block (Complete Heart Block)
Mechanism: No impulses from the atria reach the ventricles due to complete conduction block.
ECG: Atria and ventricles beat independently (AV dissociation).
Causes: Acute myocardial infarction, degenerative fibrosis, Lyme carditis, sarcoidosis, or congenital.
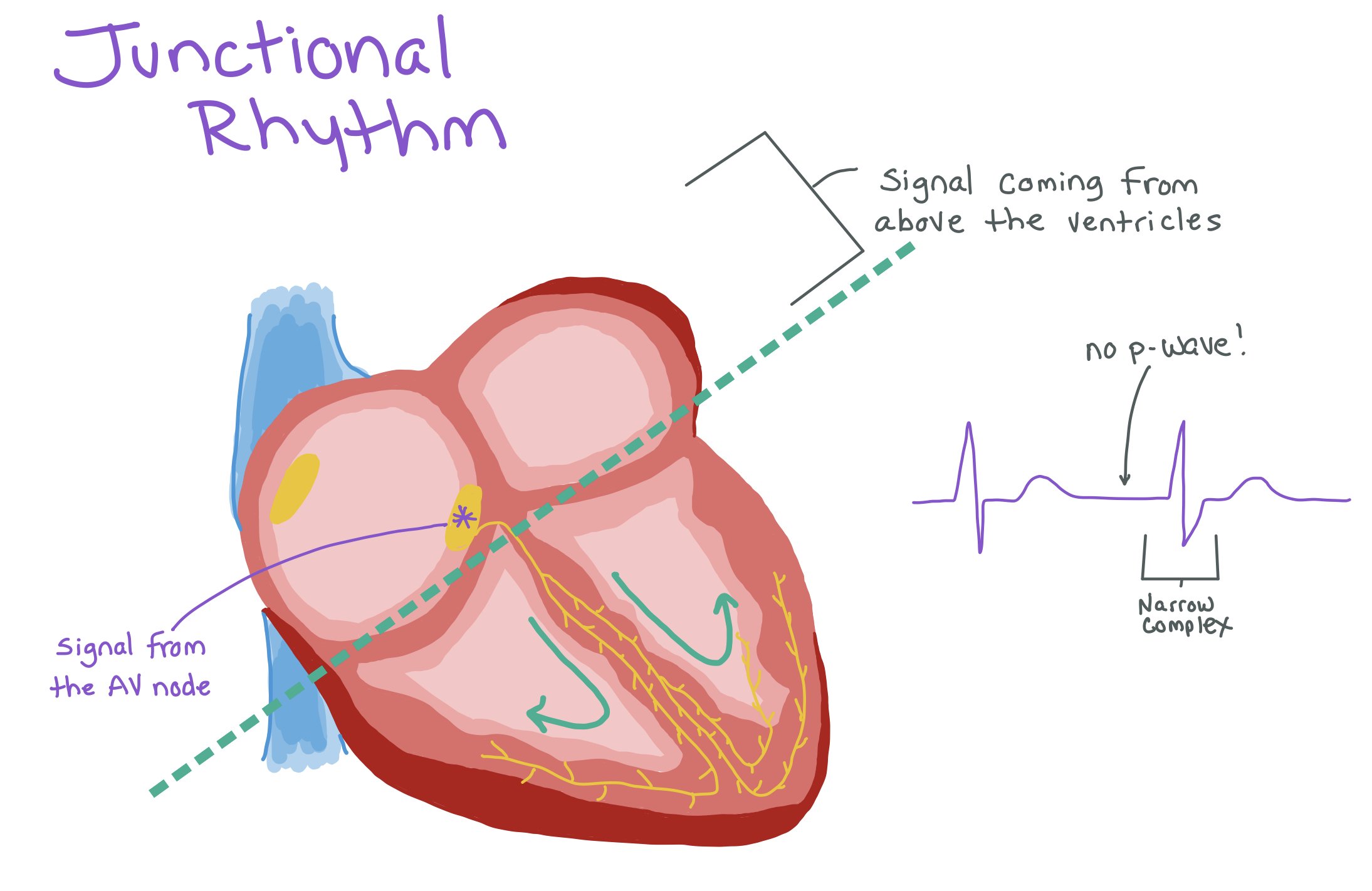
Junctional Bradycardia
Definition: A slow rhythm originating from the AV junction (near the AV node) when the SA node fails to fire or conduct.
When this occurs, patients tend to not have a p-wave (as the rhythm is not coming from the SA node) but still have narrow QRS complexes as the signal is still coming from above the ventricles.
Mechanism: The AV junction takes over as the heart’s pacemaker, but its intrinsic rate (40–60 bpm) is slower than the SA node.
Causes: SA node disease (e.g., Sick Sinus Syndrome), increased vagal tone, ischemia, digitalis toxicity, or hypothyroidism.
ECG Characteristics: Absent or inverted P waves (as the rhythm is not originated from the SA node), narrow QRS complexes (as the pacing signal is still coming from above the ventricles), and a slow, regular rhythm.
Within the heart, there are multiple pacemakers that can each pace at a different rate. Normally, the SA node paces the heart at a rate of 60-100 beats per minute. If the SA node stops working appropriately, the AV node will start pacing the heart (usually at a rate of 45-50 beats per minute). If the AV node stops working, the Bundle of His will pick up the pace, and so on.
Sometimes, it can be difficult to distinguish Mobitz I vs Mobitz II. The easiest way to do this is to look at the PR-interval before and after the dropped beat. For Mobitz I, the PR- interval will be shorter after the dropped beat. For Mobitz II, the PR-interval will be the same before and after!!
In this picture, you can see that this rhythm has no p-waves but has a narrow complex, suggesting that the signal is coming from above the ventricles but not the sinus node.
Note: When patients have 1st, 2nd, or 3rd degree heart block, the p-waves should march out!!! If they don’t, you may need to rethink the diagnosis!!
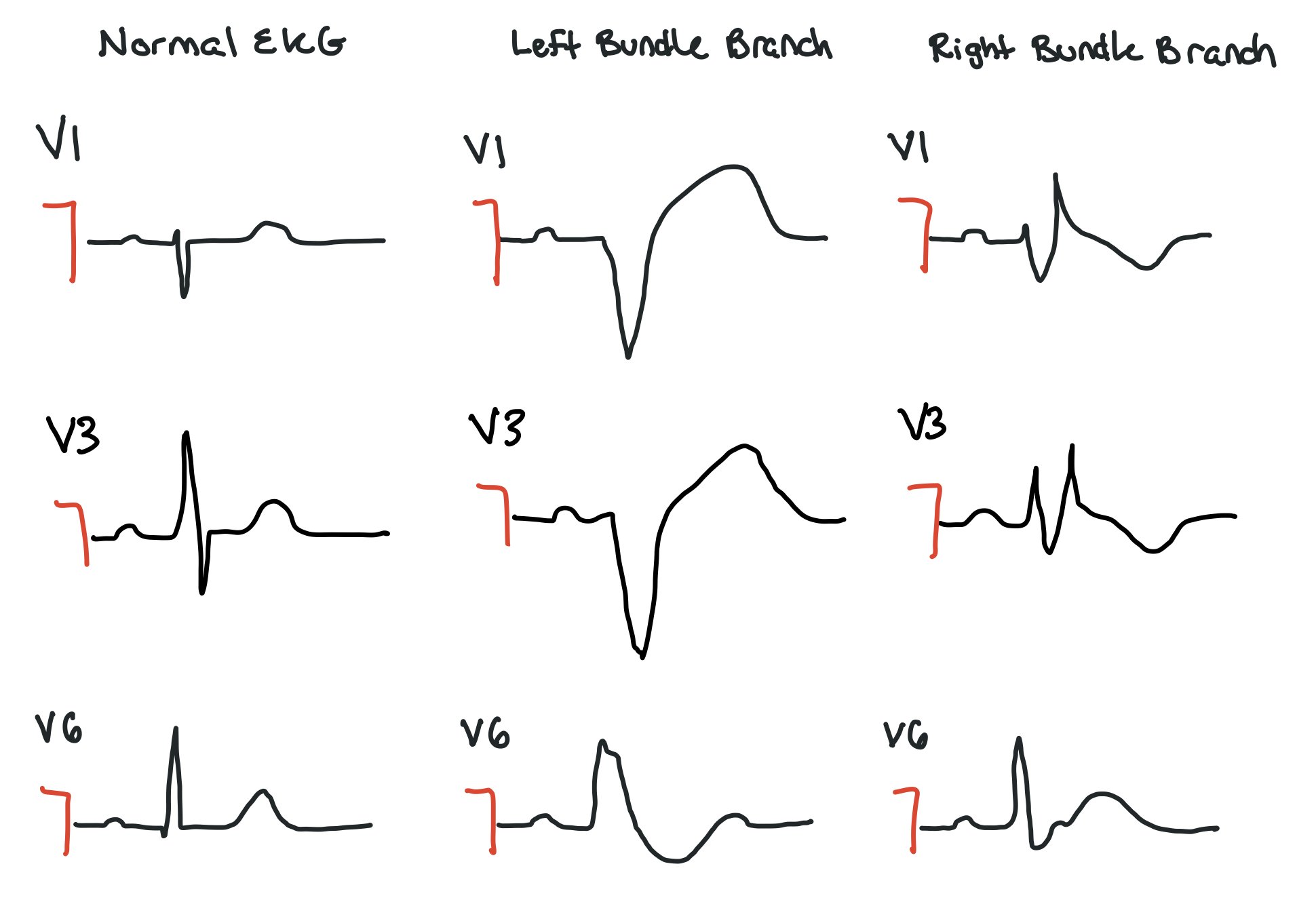
Bundle Branch Blocks
This picture compares the precordial leads in a normal EKG vs ones with left bundle or right bundle branch blocks. Normally, we expect a thin complex that is pointing down in V1 and progressively starts to point up as it gets to V6. When a patient has a bundle branch block, the complex will be wider.
Right Bundle Branch Block (RBBB)
Pathophysiology:
Right bundle branch is delayed or fails to conduct the impulse, while the left bundle functions normally.
The left ventricle (LV) depolarizes first, followed by delayed right ventricular (RV) activation via muscle-to-muscle conduction.
Often associated with pulmonary hypertension, congenital heart defects (e.g., atrial septal defect), or right ventricular strain.
ECG Criteria:
QRS duration ≥120 ms
rsr′, rsR′, or rSR′ in leads V1–V2 (“M-shaped” R′)
Wide, slurred S waves in leads I, V5, and V6
Secondary ST-T changes in right precordial leads
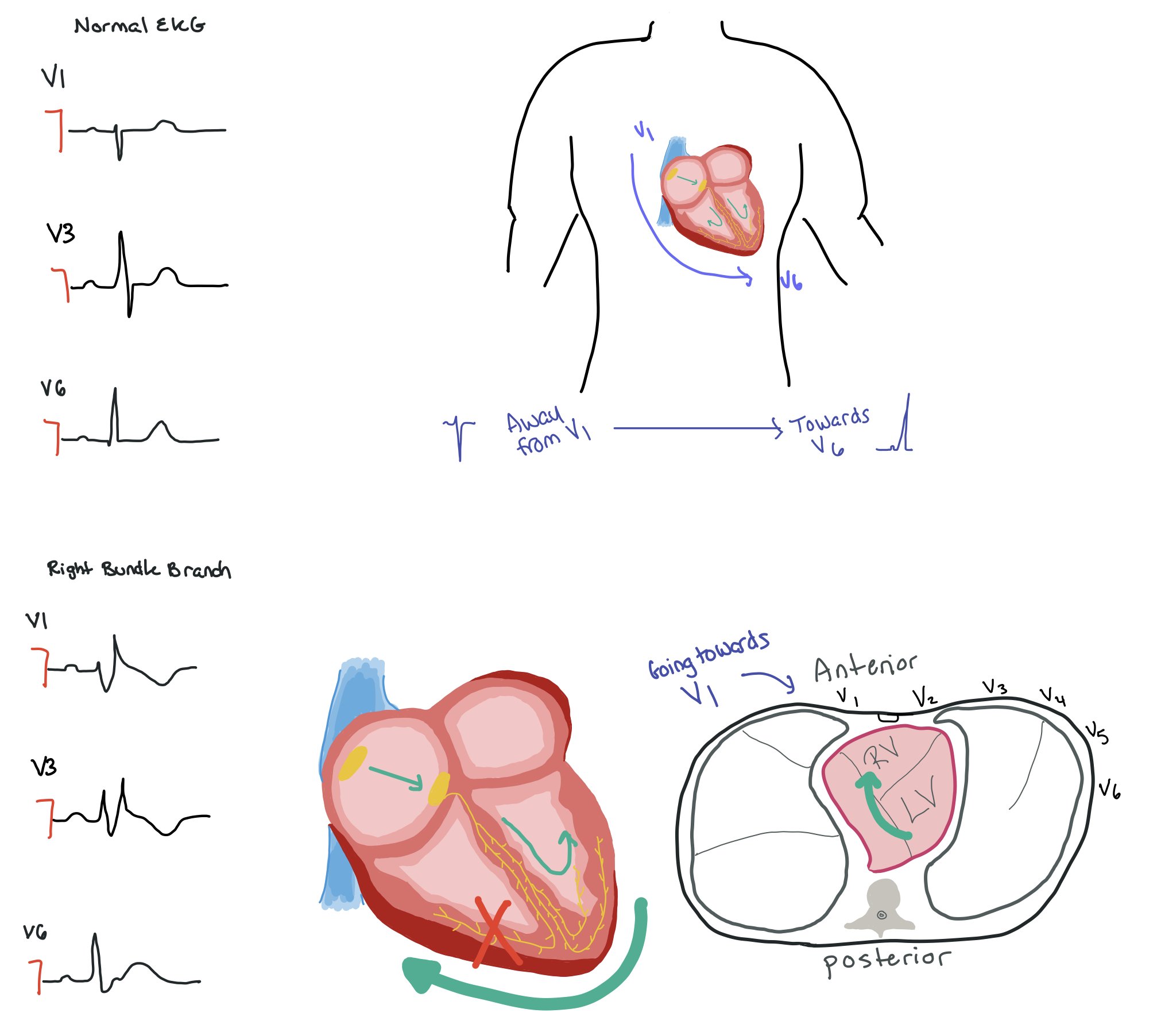
Normally, the signal is moving away from V1 → V6. From a vector standpoint, this means that in a normal heart with correctly placed leads, the signal should be negative in V1 and become more positive as it moves towards V6. In a RBBB, the signal goes from the SA node → LBBB and then has to move towards the right side of the heart. If you think about what the electricity is actually doing (as seen in the picture of the cross-sectional view of the chest), the signal is moving from the left side of the heart towards the right side. As such, the signal is going towards V1 so it will be positive.
Left Bundle Branch Block (LBBB)
Pathophysiology:
The left bundle branch fails to conduct impulses, so the RV activates first, followed by delayed LV depolarization, disrupting normal intraventricular synchrony. Patients with LBBB have higher probabilities of having underlying structural heart disease and LV dysfunction, so these patients should be evaluated with a TTE.
Associated with hypertension, ischemic heart disease, dilated cardiomyopathy, or fibrosis of the conduction system.
ECG Criteria:
QRS duration ≥120 ms
Broad, notched or slurred R waves in leads I, aVL, V5, and V6
Absent Q waves in lateral leads
Deep S waves in V1–V3
Discordant ST-T changes (ST depression and T wave inversion opposite QRS direction)
In a LBBB, the signal moves from the right side of the heart towards the left side. When looking at the cross sectional view, the signal is still going away from V1 and towards V6. As such, V1 is still negative, while V6 is positive.
Workup for Bradycardia
A thorough evaluation includes:
History and Physical Examination
Symptoms: Syncope, fatigue, lightheadedness, palpitations, or chest pain.
Important factors to consider is frequency, timing, duration, severity, triggers, and alleviating factors
Medication review and assessment for reversible causes. Common medications include beta blockers, calcium channel blockers, and even medications like Donepezil (see below for a more comprehensive list from the AHA/ACC/HRS 2018 Bradycardia Guidelines).
Electrocardiogram (ECG)
Identifies rhythm type, conduction delays, or ischemic changes.
Also look for signs of electrolyte derangements, such as hyper- or hypokalemia!
Laboratory Tests
Evaluate for reversible causes:
Ischemia
Metabolic (think acidosis, hypoxia, hyperkalemia, hypokalemia, and hypothermia)
Thyroid dysfunction
Infections (such as endocarditis, Lyme carditis, Chagas disease)
Systemic and infiltrative diseases— due to their direct or indirect impact on the sinoatrial (SA) node, atrioventricular (AV) conduction system, or autonomic nervous regulation. Therefore, make sure to also rule out diseases like amyloidosis and sarcoidosis, as well as rheumatologic conditions like rheumatoid arthritis, scleroderma, lupus, and systemic sclerosis.
Is the bradycardia occurring during sleep? If so, consider getting a sleep study to assess for sleep apnea! Treating the sleep apnea will help treat the bradycardia in many cases!
Imaging and Advanced Diagnostics
TTE: Evaluating for structural abnormalities, such as older congenital defects or new vegetations or valvular abnormalities. Bradycardia is a common complication post-TAVR as the valve can push on the conduction system.
Exercise EKG: Can do with patients with suspected chronotropic incompetence
Cardiac MRI: Assessing for ischemia or infiltrative disease
Cardiac biopsy: Done in cases when there is a concern for infiltrative disease, such as sarcoid or amyloid.
Left Heart Cath: Looking for areas of decreased perfusion / flow which can suggest ischemia
6. Determining if the patient has chronotropic incompetence
When people exert themselves, their heart rates should increase to keep up with the metabolic demand of that activity. The literature specifically defines this as failure to meet 80% of the expected age-predicted maximal HR (220 - age).
In patients who have baseline bradycardia, we can calculate the HR reserve, which is the expected HR (220 - age) - resting HR. Age, sex, and presence of other comorbidities should also be taken into account when making this calculation; however, its important that we consider the patient in front of us and their symptoms.
This table from the AHA/ACC/HRS 2018 Bradycardia Guidelines provides a more comprehensive list of medications that can cause bradycardia.
Management of Bradycardia
When to Be Concerned About Bradycardia
Bradycardia requires time-sensitive attention when:
Symptomatic Presentation
Symptoms of decreased cardiac output, such as syncope, hypotension, or altered mental status.
High-Risk Features on ECG
Mobitz II, high grade AV block, or third degree heart block — almost ALWAYS require permanent pacing
Prolonged asystolic pauses (>3 seconds)
Ventricular escape rhythms.
Association with Reversible Causes
Acute myocardial infarction, severe electrolyte derangements, endocarditis, or drug overdose.
*** All bradycardia should be worked up!
Note: there is no specific HR minimum or pause duration that warrants pacing on its own when the patient is not in heart block; rather, we look at the whole picture and take the patient’s symptoms into account when determining whether or not they need to be permanently paced.
Management Strategies
Acute Setting
Pharmacologic Interventions
Atropine: First-line agent for symptomatic bradycardia (0.5 mg IV every 3-5 minutes; max 3 mg).
Epinephrine (2-10 mcg/min) or Dopamine (5 mcg/kg/min with increments 5 mcg/kg/min every 2 min up to maximum of 20 mcg/kg/min): For atropine-refractory cases; acts as positive chronotropes.[5]
Temporary Pacing
Transcutaneous Pacing: Quick, non-invasive; applied via defibrillator pads (should be short in duration because it’s painful and really should only be for hyperacute settings)
Transvenous Pacing: More invasive but reliable; requires central venous access.
The picture above shows a depiction of a patient with a temporary transvenous pacer (TVP),
These pictures depict some of the most commonly used cardiac monitors.
Top left: Depicts a patient with a Holter Monitor, which is an external monitor that patients usually wear for 24-48 hrs (although some can now be warn for up to 2 weeks). The Holter Monitor captures every heart beat as it is a continuous monitor. This is a good monitor to choose for patients who are having very frequent symptoms.
Top right: The Loop Recorder is an implantable device ~1-2 inches small that will continuously record every heart beat. The device will then “loop” over and record over data that does not include any events (think recording over an old VCR tape). This device usually lasts up to 4 years, which makes it a great option for people who have very infrequent events.
Bottom middle: The external event monitor can be a patch that can record and transmit data. Only significant arrhythmias and patient triggered events are detected. This device is usually worn for ~30 days. Choose this device for patients who have relatively frequent events and are very aware of their symptoms.
Table depicting cardiac monitor types and their differences:
Device Therapy for Bradycardia
Indications for Permanent Pacemakers
Symptomatic bradycardia unresponsive to reversible cause correction, such as revascularization in MI, medications (atropine, dopamine and epi), electrocytes repletion.
Mobitz II, high-grade AV blocks, third degree heart block
Symptomatic sinus node dysfunction (SND) (e.g., sick sinus syndrome).
Device Types
Single-Chamber Pacemakers: Stimulate either the atrium or ventricle.
The MOST trial (2002) showed that atrial-only pacing often failed over time in patients with sinus node dysfunction because they developed AV block, needing ventricular pacing later.
Fellow Pearl: Single-chamber pacing is now more rare because studies have shown sinus node dysfunction generally predicts AV nodal disease. New devices like the Micra (by Medtronic) are leadless and placed directly in the right ventricle are sometimes placed used when only occasional ventricular pacing is needed-such as: Rare AV block, When traditional leads are risky (e.g., infection risk or no vein access)
Dual-Chamber Pacemakers: Coordinate atrial and ventricular contractions. Table below summarizes the differences
Cardiac Resynchronization Therapy (CRT): When patients have an EF between 35-50% and are expected to require ventricular pacing >40% of the time, CRT (or His ) pacing indicated to prevent progression to heart failure.
The cartoon picture above shows a CRT with the RA lead (pink), RV (green), and LV lead (blue). Use this picture to compare to the CXR on the left to help you visualize where the leads are and how they are placed in the chest.
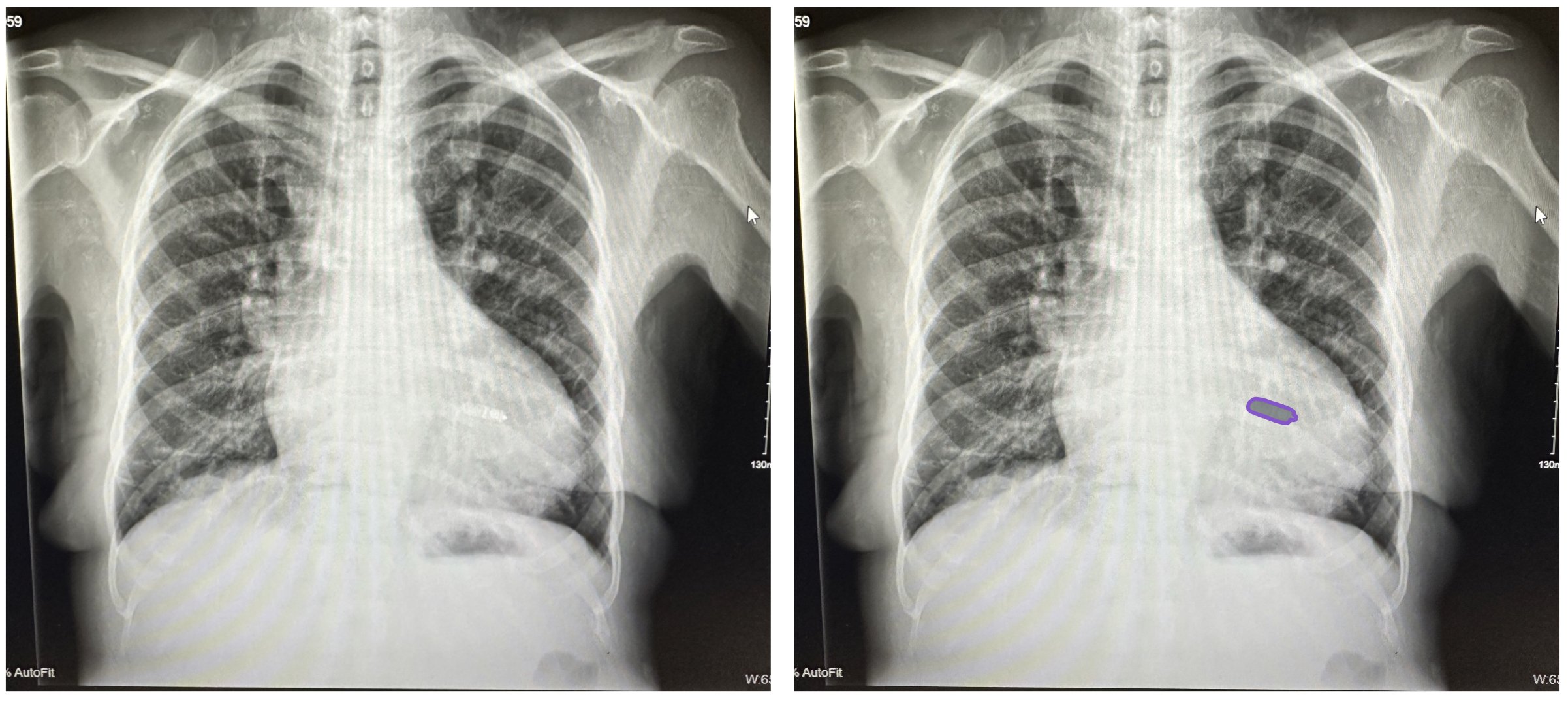
CXR with a patient who has a leadless device.
The above picture shows a leadless device (as opposed to the picture below which shows a cardiac resynchronization device —CRT)
Summary
Bradycardia requires a systematic approach to differentiate types, assess underlying causes, and identify severity. Acute management focuses on stabilizing the patient using pharmacologic agents and temporary pacing, while long-term care often involves pacemaker implantation. Early recognition and treatment are pivotal to prevent complications and improve patient outcomes..
Management of Bradycardia per the 2020 American Heart Association Guidelines.
Back to the Case:
1. How do you differentiate between the types of bradycardia, and how do you work it up?
History is key!!! Being able to differentiate frequency, timing, duration, severity, triggers, and alleviating factors can help determine the type of bradycardia. Next, it’s important to determine if the patient is symptomatic or not. If possible, get an EKG to help determine if the bradycardia is due to a sinus or AV nodal dysfunction or something distal in the conduction system. If the EKG does not pick up any abnormalities, the next step may be to have the patient wear a cardiac monitor (inpatient vs outpatient depending on symptoms and hemodynamic stability). TTEs, stress EKGs, cardiac MRIs can be used to help further evaluate the bradycardia if needed. Make sure to walk the patient and to ensure they do not have chronotropic incompetence!! Reviewing the patient’s medication, as well as getting labs to rule out thyroid dysfunction, acute MI, infection, metabolic derangements, and infiltrative or systemic diseases.
2. When do we get concerned about bradycardia?
Bradycardia becomes concerning if patients are symptomatic, do not have good chronotropic response to activity, or have high risk features seen on their EKG (i.e. Mobitz II, high degree AV block, or third degree heart block). We need to ensure we do an appropriate workup before discharging these patients.
3. What do we do in the acute setting (pharm vs transvenous / transcutaneous pacing) vs long term?
If patients are unable due to their bradycardia, the first line agent should be atropine. If ineffective, we can escalate to dopamine or epinephrine drips. Patients who are still bradycardic will most likely require transcutaneous (acute) pacing until they can be given a transvenous pacer.
Acute stable: Try atropine first; if ineffective, escalate to dopamine or epinephrine infusion.
Acute unstable: Use transcutaneous pacing immediately, followed by transvenous pacing if needed.
Long-term: Treat reversible causes; if persistent or symptomatic, consider permanent pacemaker.
4. When do people get devices? What type of devices do they usually get?
Patients get devices for symptomatic bradycardia, Mobitz II or 3rd-degree AV block, or sick sinus syndrome.
Dual-chamber pacemakers (RA + RV) are most common.
CRT devices (CRT-P or CRT-D) are used in patients with HF and QRS >120 ms. When patients have an EF between 35-50% and are expected to require ventricular pacing >40% of the time, CRT (or His ) pacing indicated to prevent progression to heart failure.
ICDs are used for arrhythmia prevention in high-risk patients (e.g., sarcoid, low EF).
Further Learning:
Resident Responsibilities
When called about bradycardia, make sure to get an EKG to determine where in the conduction system the issue is occurring. Patients with Mobitz II, high-grade AV block, and third degree heart block will always need a device!
History is key!!! Being able to determine frequency, timing, duration, severity, triggers, and alleviating factors can help determine the type of bradycardia.
Any time a patient is suspected to have bradycardia with sleep, a sleep screen for sleep apnea should be conducted!!
Remember that patients can be bradycardic and NOT be symptomatic!!
If they are unstable, pacing is always an option. If stable, try atropine, if still symptomatic and brady, try dopamine or epinephrine
Attending Pearls
Make sure to assess for carotid sinus sensitivity! When patients have this, they are extremely sensitive to anything touching their neck, such as ties and tight collars. Sometimes doing things such as shaving or turning your head in the car to look over your shoulder can compress the carotid sinus and activate vagal reflexes! To assess for this, light carotid massage can be performed on one side of the neck. If the patient becomes symptomatic, they may have carotid sinus sensitivity! Before performing this procedure, make sure to auscultate the carotid sinus for abnormalities (such as a bruit) as strokes have been reported s/p carotid massage.
How’d we do?
The following individuals contributed to this topic: Nonye Uche, MD; Aamir Twing, MD; Mark McCauley, MD, PhD
Resources
Manoj, P., Kim, J. A., Kim, S., Li, T., Sewani, M., Chelu, M. G., & Li, N. (2022). Sinus node dysfunction: current understanding and future directions. AJP Heart and Circulatory Physiology, 324(3), H259–H278. https://doi.org/10.1152/ajpheart.00618.2022
Kashou, A. H., Goyal, A., Nguyen, T., Ahmed, I., Chhabra, L., & Kukuc, L. G. (2024, February 12). Atrioventricular Block (Nursing). StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/sites/books/NBK568758/
Hafeez, Y., & Grossman, S. A. (2023, February 5). Junctional rhythm. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK507715/
Buttner, E. B. (2024, October 8). Left Bundle Branch Block (LBBB). Life in the Fast Lane LITFL. https://litfl.com/left-bundle-branch-block-lbbb-ecg-library/
Uptodate: Evaluation and management of bradycardia and atrioventricular (AV) block in adults
– Authors: Joseph J. Brady, MD; Peter J. Zimetbaum, MD
– Accessed March 2025.
https://www.uptodate.comLamas, G. A., Lee, K. L., Sweeney, M. O., Silverman, R., Leon, A., Yee, R., Marinchak, R. A., Flaker, G., Schron, E., Orav, E. J., Hellkamp, A. S., Greer, S., McAnulty, J., Ellenbogen, K., Ehlert, F., Freedman, R. A., Estes, N. M., Greenspon, A., & Goldman, L. (2002). Ventricular pacing or Dual-Chamber pacing for Sinus-Node dysfunction. New England Journal of Medicine, 346(24), 1854–1862. https://doi.org/10.1056/nejmoa013040
Rehorn, M. R., Loungani, R. S., Black-Maier, E., Coniglio, A. C., Karra, R., Pokorney, S. D., & Khouri, M. G. (2020). Cardiac implantable Electronic devices. JACC. Clinical Electrophysiology, 6(9), 1144–1154. https://doi.org/10.1016/j.jacep.2020.04.020
Kusumoto, F. M., et al. (2019).
2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay.
Journal of the American College of Cardiology, 74(7), e51–e156. https://doi.org/10.1016/j.jacc.2018.10.044